Abstract
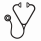
Introduction: Sickle cell disease (SCD) is characterized by unpredictable, recurrent acute and chronic persistent pain, and progressive organ damage leading to substantial morbidity, impaired quality of life and increased healthcare utilization. Hematopoietic cell transplantation (HCT) is the only therapy for SCD with curative intent. HCT is associated with excellent overall and event free survival especially in young children undergoing HCT from HLA identical family donors. Given these outcomes and improved supportive care, HCT is increasingly being offered to adults with SCD. However, a paucity of data exists on health related quality of life (HrQoL) in patients who are long term survivors of HCT. Therefore, a clear need exists to study HrQoL in children and adults post HCT to determine the long term benefit of HCT for SCD. We report the results of an interim analysis of HrQoL in recipients of HCT for SCD.
Methods: We enrolledchildren and adults with SCD ≥ 1-year post-HCT in the Sickle Cell Transplant Evaluation of Long Term and Late Effects Registry (STELLAR). We obtained patient self-report of health status and health practices using a web based platform. Individuals under age 18 years and/or proxies completed Patient Reported Outcome Measurement Information System (PROMIS) version 25. Individuals over age 18 completed PROMIS version 29. Surveys were completed at enrollment and annually thereafter. T-scores were calculated for each survey domain. HrQoL in children (PROMIS25) and adults (PROMIS29) was compared to age appropriate norms (mean 50 ± 10). Univariate analysis was performed by age, gender, marital status, time from transplant, history of preHCT stroke, frequency of pain, number of emergency department (ED) visits, length of stay, income, and education assessed at the time of PROMIS survey administration. Statistical analysis was conducted using SAS Version 9.4. Descriptive statistics for each variable were reported. For numeric covariates, the mean and standard deviation (SD) were calculated and presented with frequency and its percentage shown for category variables. For repeated measures, generalized estimating equation (GEE) model was performed to detect the association. The significance level was set at 0.05.
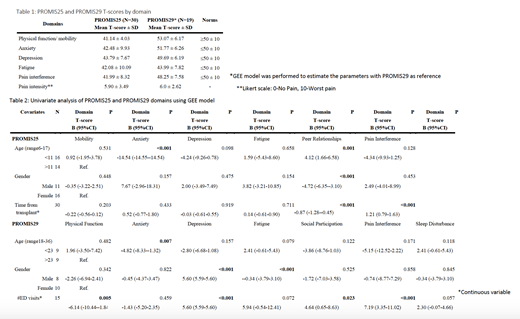
Results: Twenty-seven individuals completed 30 PROMIS25 surveys and 15 individuals completed 19 PROMIS29 surveys from 2017-2021. The mean age at transplant was 11.4years (SD 3.1) and 23.8 years (SD 6.2) in adults, respectively. Sixty percent and 50%, respectively, were female. The mean time from transplant was 4.2 years (SD 3.9) and 9.1 years (SD 7.1), respectively. Physical function/mobility, anxiety, depression, fatigue, and pain T-scores for each PROMIS measure are reported below in Table 1. Univariate analysis of PROMIS25 T-scores showed a statistically significant difference in anxiety by age and peer relationships by age, gender, and time from transplant. Time from transplant also had a statistically significant association with pain interference. PROMIS29 T-scores also showed a statistically significant difference in anxiety by age. Gender had a statistically significant association with depression and fatigue. Number of ED visits had a significant association with physical function, depression, social participation, and pain interference (Table 2). Other covariates lacked a statistically significant association or had insufficient sample sizes for comparison.
Conclusions: Long-term HrQoL after HCT for SCD is within population norms for adults and children, though children have better HRQoL compared to adults. Better scores in females suggests the need for study of gender as a mediator of HrQoL outcomes. The observed relationship between HrQoL domains and education, income, time from transplant, and number of ED visits, a surrogate for healthcare utilization, suggest the need for careful study of these and other factors that can influence HrQoL post HCT over the long-term. In addition, further research in patient reported outcomes has the potential to identify needed areas of intervention and surveillance for this population.
Guilcher: Project Sickle Cure Study: Other: Principal Investigator, Research Funding; BlueBirdBio: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract